Glenmark brings relief with FabiFlu, priced @ Rs 103 a tablet
By MYBRANDBOOK
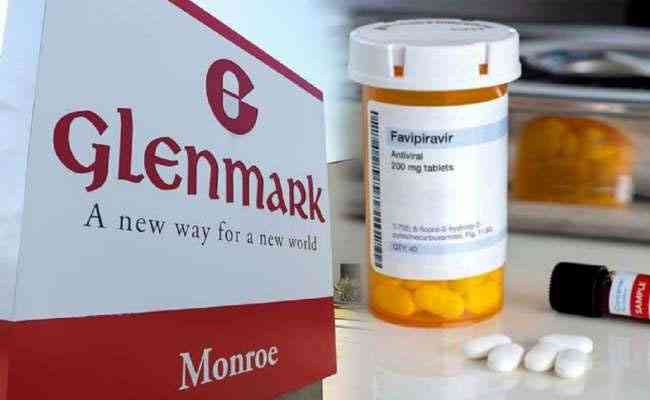
Glenmark developed active pharmaceutical ingredient and formulation for FabiFlu through in-house R&D; DCGI allowed fast track trials with Phase III in limited patients; approval process is also under Emergency Use Authorisation. The drug acts by getting into cells and inhibit the activity of viral replication to reduce the viral load.
Glenmark Pharmaceuticals become the first Indian pharma company to commercially launch an antiviral drug - Favipiravir with brand name FabiFlu - for the treatment of mild to moderate COVID-19 patients. The company received marketing and manufacturing approval from the Indian drug regulator, Drug Controller General of India (DCGI) and launched the product in the Indian market today.
Priced at Rs 3,500 for a pack of 34 tablets (Rs 103 per tablet), the dosage is 200 mg X 9 tablets on the day one and 200 mg X 4 tablets a day for 14 days of the treatment. Glenmark did a clinical trial among 90 mild and 60 moderate COVID-19 patients across 11 sites in India. The drug is claimed to have an efficacy of over 80% in the treatment of COVID-19 mild to moderate patients.
Sources said Delhi-based Brinton Pharmaceuticals, Bengaluru-based Strides Pharma, Mumbai-based Lasa Supergenerics, Hyderabad-based Optimus Pharma are some of the other Indian firms that have applied for approval and are readying its launch in India.
Key Highlights :
· Glenmark first pharma firm to get approval for an oral drug to treat COVID-19 patients in India
· Priced at Rs 3,500 for 34 tablets, the dosage is 200 mg X 9 tablets on day one and 200 mg X 4 tablets a day for 14 days
· Global trials show the efficacy of over 80-88%; Japan, Bangladesh and UAE already use the drug for COVID-19 treatment
· Strides Pharma, Brinton Pharmaceuticals, Lasa Supergenerics and Optimus Pharma among firms readying its launch
· API and formulation developed by Glenmark's in-house R&D unit
As per the report,Glenmark had developed the active pharmaceutical ingredient (API) and the formulation for FabiFlu through in-house R&D. The DCGI allowed fast track trials with Phase III in limited patients. The approval process is also under Emergency Use Authorisation (EUA).
"The approval comes at a time when cases in India are spiralling like never before and putting tremendous pressure on our healthcare system. FabiFlu will reduce this pressure. Glenmark will work with the government and medical community to make it quickly accessible to patients across the country," said Glenn Saldanha, Chairman and Managing Director, Glenmark Pharmaceuticals.
The drug acts by getting into cells and inhibit the activity of viral replication to reduce the viral load. A high rate of viral replication can be controlled with early use of antiviral drugs. In later stages, viral replication slows down and the body's violent immune response drives disease to complications and organ failure, said the company sources.
Glenmark is also undertaking a study combining two anti-viral drugs, Favipiravir (an approved drug for novel flu pandemics) with Umifenovir (an approved drug for Influenza) in COVID-19 patients.
Favipiravir, sold under the brand name Avigan by Fujifilm Toyama Chemical and approved in Japan since 2014 in treating influenza, is already being used commercially in the therapeutic management of COVID-19 in Bangladesh and UAE.
It is under approval process in Egypt and Jordan and is a part of the treatment protocol in Russia, Japan and Saudi Arabia. About 18 global clinical trials in 3,000 subjects are going on including in India, USA, Canada, Italy, China, France, UK and other countries.


Nazara and ONDC set to transform in-game monetization with ‘
Nazara Technologies has teamed up with the Open Network for Digital Comme...

Jio Platforms and NICSI to offer cloud services to government
In a collaborative initiative, the National Informatics Centre Services In...

BSNL awards ₹5,000 Cr Project to RVNL-Led Consortium
A syndicate led by Rail Vikas Nigam Limited (abbreviated as RVNL), along wi...

Pinterest tracks users without consent, alleges complaint
A recent complaint alleges that Pinterest, the popular image-sharing platf...


Icons Of India : B.V.R. Subrahmanyam
A 1987 batch (Chhattisgarh cadre) Indian Administrative Service Office...

Icons Of India : NEERAJ MITTAL
He started his career as an IAS Officer in 1992. He has held various a...

ICONS OF INDIA : SOM SATSANGI
With more than three decades in the IT Sector, Som is responsible for ...

CERT-IN - Indian Computer Emergency Response Team
CERT-In is a national nodal agency for responding to computer security...

PFC - Power Finance Corporation Ltd
PFC is a leading financial institution in India specializing in power ...

NIC - National Informatics Centre
NIC serves as the primary IT solutions provider for the government of ...


Indian Tech Talent Excelling The Tech World - Soni Jiandani, Co-Founder- Pensando Systems
Soni Jiandani, Co-Founder of Pensando Systems, is a tech visionary ren...

Indian Tech Talent Excelling The Tech World - Sundar Pichai, CEO- Alphabet Inc.
Sundar Pichai, the CEO of Google and its parent company Alphabet Inc.,...

Indian Tech Talent Excelling The Tech World - JAYASHREE ULLAL, President and CEO - Arista Network
Jayshree V. Ullal is a British-American billionaire businesswoman, ser...